Human Ophthalmic Imaging with 1060nm Swept Source OCT
Glaucoma, a world leading cause of irreversible blindness, is a progressive neuropathic disease often associated with uncontrolled Intra-Ocular Pressure (IOP). Glaucomatous optic neuropathies pertain to the posterior deformation of the Optic Nerve Head (ONH) and excavation beyond the anterior scleral canal opening. The lamina cribrosa is a sieve-like collagenous membrane that provides structural and nutritional support to the nerve fibre bundles that sits at the base of the ONH and inserts into the scleral canal wall. Although the pathophysiology of glaucomatous damage is multi-factorial, several studies in models of early experimental glaucoma in non-human primates implicated that morphological changes of the lamina cribrosa and sclera canal wall is central in the pathogenesis of glaucomatous optic neuropathy.1-3 Quantitative analysis of these deformations through in vivo imaging may provide predictive information on an individual's susceptibility to early glaucomatous damage to a given level of IOP.
Non-invasive ophthalmic medical image acquisition and analysis can provide a path to early diagnosis and management of glaucoma. However, imaging of the retina and ONH is complicated by their location at the back of the eye. Optical Coherence Tomography (OCT) is a non-contact, non-invasive medical imaging modality that can acquire sub-surface high-resolution cross sectional images of biological tissue 4. It provides images, approaching that of histology, at depths up to 2-3 mm below the surface sufficient for morphometric analysis.
ONH Imaging
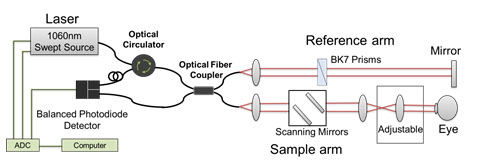
We have developed a 1060nm Swept Source (SS) OCT system (Figure 1 ) suitable in a clinical environment. The longer wavelength enables deeper penetration into the ONH and results in better visualization of the lamina cribrosa scleral wall (Figure 2).
Visualization and Analysis
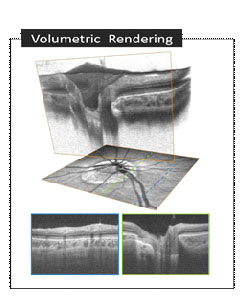
The acquired image is processed and visualized in 3D space in whole-volume as well as in arbitrary cross-sections, allowing a powerful intuitive representation of the data for qualitative evaluation of the ONH morphology (Figure 2).
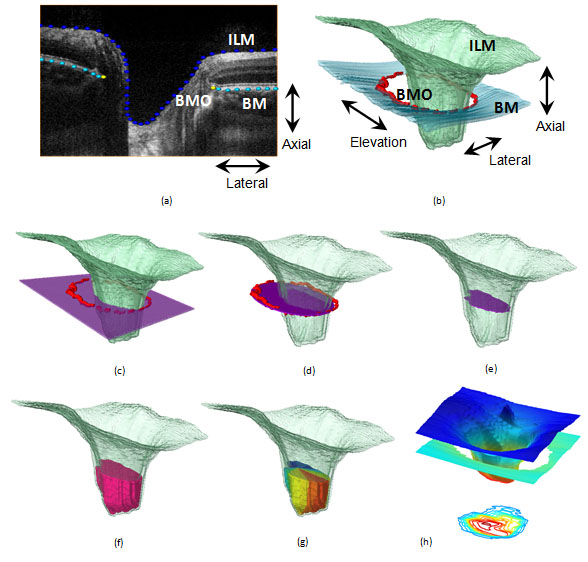
is the first step in quantitative shape analysis . This is currently carried out by trained raters who manually segment the target structures: Inner Limiting Membrane (ILM), Retinal Nerve Fibre Layer (RNFL), Bruch's Membrane (BM), and Bruch's Membrane Opening (BMO) (Figure 3-a).
The deeper tissue penetration of the 1060nm light source will facilitate segmentation of scleral wall, Anterior Lamina Cribrosa Surface (ALCS), and anterior lamina cribrosa Insertion Points (IP). Preliminary analysis was performed in myopic eyes in which the shallow optic cups allow visualization of the deeper structures using 830 nm light source (Figure 4, Figure 5).4
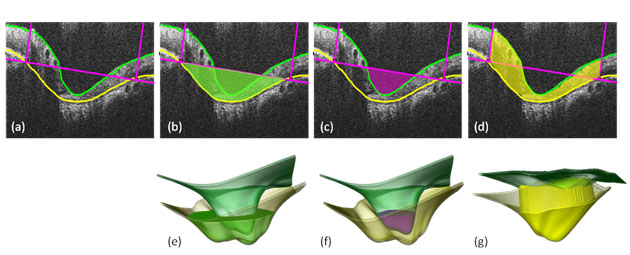
From the segmented points various morphological parameters of interest are computed, such as retinal layer thicknesses, volumes, areas, distances between structures, and obliqueness (Figure 3).5 These morphometrics allow quantitative measurement and sensitive detection of structural deformations and variability, and enable rigorous longitudinal and cross-sectional comparison and statistical analysis.
References
1 Burgoyne, C.F., et al., The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res, 2005. 24(1): p. 39-73.
2 Yang, H., et al., 3-D histomorphometry of the normal and early glaucomatous monkey optic nerve head: prelaminar neural tissues and cupping. Invest Ophthalmol Vis Sci, 2007. 48(11): p. 5068-84.
3 Yang, H., et al., 3-D histomorphometry of the normal and early glaucomatous monkey optic nerve head: lamina cribrosa and peripapillary scleral position and thickness. Invest Ophthalmol Vis Sci, 2007. 48(10): p. 4597-607.
4 Lee, S., et al., End-to-end pipeline for spectral domain optical coherence tomography and morphometric analysis of human optic nerve head. J Med Biol Eng, 2011. 31(2): p. 111-120.
5 Lee, S., et al., Morphometry of the myopic optic nerve head using FDOCT. Proc SPIE 7889, 788932, 2011.